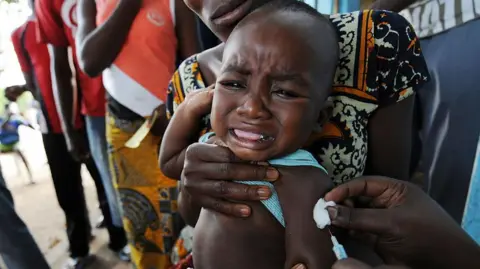
WHO Condemns US-Funded Vaccine Trial on Newborns in Guinea-Bissau
The World Health Organization (WHO) has criticized a now-halted plan to run a hepatitis B vaccine trial involving thousands of newborns in Guinea-Bissau as unethical. This US-funded study intended to administer the vaccine to one group of babies at birth, while delaying the vaccination for another group until they were six weeks old.
WHO expressed significant concerns regarding the trial, describing the birth-dose hepatitis B vaccine as an effective and essential public health intervention with a proven record. The trial faced criticism for its scientific justification, ethical safeguards, and adherence to established research standards involving humans.
Led by the US health department, overseen by Robert F. Kennedy Jr., the trial aimed to investigate the broader health effects of the vaccine. However, the WHO refuted the trial's foundation, emphasizing the critical need for timely vaccination to thwart hepatitis B transmission from mothers to their infants, stating that vaccination prevents the virus in 70-95% of cases when given at birth.
Health officials in Guinea-Bissau, where more than 12% of the adult population is estimated to have chronic hepatitis B, ultimately suspended the trial following public backlash. Critics, including the former health minister of Guinea-Bissau, have argued that using babies in this study was unacceptable, emphasizing that local populations should not be treated as test subjects.
Kennedy’s department had also recently altered the US vaccination guidelines regarding hepatitis B for newborns. Despite his claims of supporting vaccination, he has faced scrutiny for promoting debunked theories related to vaccine safety.
In Guinea-Bissau, a birth dose of the hepatitis B vaccine is currently scheduled at six weeks but is anticipated to become mandatory at birth nationwide by 2028. The WHO has stated it will aid in expediting this implementation, in line with global standards.