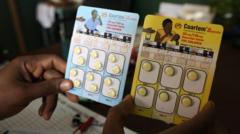
The first malaria treatment tailored for infants and very young children has officially been approved, a monumental step expected to be implemented within the coming weeks across African nations. Prior to this, the lack of approved malaria drugs for babies had forced reliance on formulations meant for older children, raising concerns of potential overdoses.
Tragic Statistics
In 2023, malaria was linked to approximately 597,000 fatalities globally, with the vast majority occurring in Africa. Alarmingly, around 75% of these deaths involved children under the age of five. Current treatments available for pediatric patients do not cater to the specific needs of infants who weigh less than 4.5 kg (approximately 10 lbs). The difference in body composition and organ maturity means that medications devised for older children could pose a risk for babies, contributing to a critical "treatment gap."
The New Hope
Developed by Novartis, the newly approved medication, branded as Coartem Baby or Riamet Baby in certain regions, has been greenlit by Swiss health authorities. The drug is poised to be distributed in malaria-endemic regions on a largely not-for-profit basis. Vas Narasimhan, CEO of Novartis, emphasizes the significance of this achievement, stating, "For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most."
Collaborative Efforts
This innovative drug was formulated in partnership with the Medicines for Malaria Venture (MMV), a not-for-profit organization based in Switzerland with initial backing from various governments, including those of the UK, Switzerland, and the Netherlands, as well as international institutions such as the World Bank. Involvement from eight African nations during the drug's assessment and trials positions them to be some of the initial beneficiaries.
Experts weigh in on the Shift
Martin Fitchet, CEO of MMV, views the approval of Coartem Baby as a substantial stride towards mitigating malaria's deadly impact on children. He stated, "With the right resources and focus, malaria can be eliminated."
Dr. Marvelle Brown, an associate professor at the University of Hertfordshire, heralds this development as a vital breakthrough in pediatric healthcare amid the high mortality rates from malaria, particularly in sub-Saharan Africa. With over 76% of malaria-related deaths occurring in children under five, this specialized treatment is seen as crucial for vulnerable demographics, including infants with compromised immune systems due to conditions like sickle cell disease.
As Novartis implements this initiative without profit motives, there are hopes that it will enhance equity in healthcare access, bolstering efforts to save lives and combat malaria's devastating effects on the youngest populations.
Tragic Statistics
In 2023, malaria was linked to approximately 597,000 fatalities globally, with the vast majority occurring in Africa. Alarmingly, around 75% of these deaths involved children under the age of five. Current treatments available for pediatric patients do not cater to the specific needs of infants who weigh less than 4.5 kg (approximately 10 lbs). The difference in body composition and organ maturity means that medications devised for older children could pose a risk for babies, contributing to a critical "treatment gap."
The New Hope
Developed by Novartis, the newly approved medication, branded as Coartem Baby or Riamet Baby in certain regions, has been greenlit by Swiss health authorities. The drug is poised to be distributed in malaria-endemic regions on a largely not-for-profit basis. Vas Narasimhan, CEO of Novartis, emphasizes the significance of this achievement, stating, "For more than three decades, we have stayed the course in the fight against malaria, working relentlessly to deliver scientific breakthroughs where they are needed most."
Collaborative Efforts
This innovative drug was formulated in partnership with the Medicines for Malaria Venture (MMV), a not-for-profit organization based in Switzerland with initial backing from various governments, including those of the UK, Switzerland, and the Netherlands, as well as international institutions such as the World Bank. Involvement from eight African nations during the drug's assessment and trials positions them to be some of the initial beneficiaries.
Experts weigh in on the Shift
Martin Fitchet, CEO of MMV, views the approval of Coartem Baby as a substantial stride towards mitigating malaria's deadly impact on children. He stated, "With the right resources and focus, malaria can be eliminated."
Dr. Marvelle Brown, an associate professor at the University of Hertfordshire, heralds this development as a vital breakthrough in pediatric healthcare amid the high mortality rates from malaria, particularly in sub-Saharan Africa. With over 76% of malaria-related deaths occurring in children under five, this specialized treatment is seen as crucial for vulnerable demographics, including infants with compromised immune systems due to conditions like sickle cell disease.
As Novartis implements this initiative without profit motives, there are hopes that it will enhance equity in healthcare access, bolstering efforts to save lives and combat malaria's devastating effects on the youngest populations.