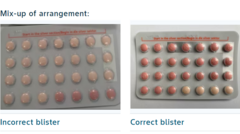
The South African Health Products Regulatory Agency has announced a precautionary recall of a specific batch of Yaz Plus contraceptive pills due to a packaging error that has raised concerns regarding contraceptive efficacy. This mix-up occurred in batch WEW96J, which is set to expire in March 2026, where several blister packs were improperly organized, resulting in the inclusion of 24 inactive pills instead of the intended hormone-filled tablets.
Manufacturing company Bayer Ltd has urged women who may have purchased packs from this batch to cease usage immediately and consult healthcare professionals. Their latest announcement emphasized the potential risk of unintended pregnancies for those who may have mistakenly taken inactive tablets while thinking they were on effective hormonal contraception.
The Yaz Plus contraceptive is designed with 24 hormone-laden pink pills, followed by four hormone-free, light orange pills. In the affected batch, several packets comprised solely of the four active pills along with 24 inactive ones, creating a significant risk for users.
Bayer has collaborated closely with regulatory agencies to conduct this recall and has highlighted that corrective measures have been taken to address the root cause of the packaging error. As a precaution, they recommend that anyone in possession of the recalled pills return them to pharmacies for refunds or replacements.
Additionally, healthcare providers, pharmacies, and wholesalers are instructed to return any packets from the identified batch. Bayer has established a helpline for consumers needing further information regarding the recall.
The company reassures patients that this incident is isolated to one specific batch, with no other products affected, as they work to maintain high standards of safety and efficacy in their contraceptive offerings.
Manufacturing company Bayer Ltd has urged women who may have purchased packs from this batch to cease usage immediately and consult healthcare professionals. Their latest announcement emphasized the potential risk of unintended pregnancies for those who may have mistakenly taken inactive tablets while thinking they were on effective hormonal contraception.
The Yaz Plus contraceptive is designed with 24 hormone-laden pink pills, followed by four hormone-free, light orange pills. In the affected batch, several packets comprised solely of the four active pills along with 24 inactive ones, creating a significant risk for users.
Bayer has collaborated closely with regulatory agencies to conduct this recall and has highlighted that corrective measures have been taken to address the root cause of the packaging error. As a precaution, they recommend that anyone in possession of the recalled pills return them to pharmacies for refunds or replacements.
Additionally, healthcare providers, pharmacies, and wholesalers are instructed to return any packets from the identified batch. Bayer has established a helpline for consumers needing further information regarding the recall.
The company reassures patients that this incident is isolated to one specific batch, with no other products affected, as they work to maintain high standards of safety and efficacy in their contraceptive offerings.